Stem Cell Engineering Research Group
SCERG is focused on Cell Bioprocessing, Biomaterials and Tissue Engineering from cell reprogramming to biomanufacturing for Regenerative Medicine, Disease Modelling, Drug Testing and Cellular Agriculture.
Keywords
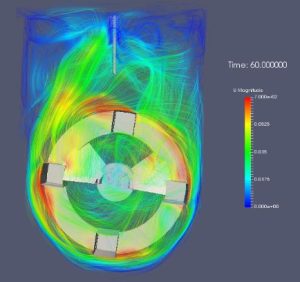
Bioreactors and bioprocesses for cells tissues and organs
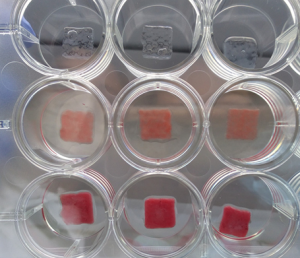
Biomaterials / 3D printing and tissue engineering
Stem cell-based therapies & regenerative medicine
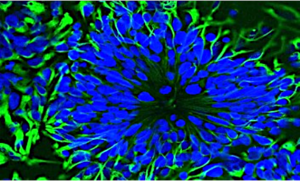
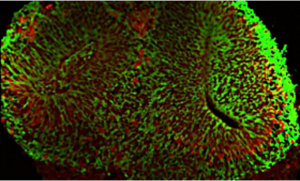
Organoids for modeling disease and development
Cell reprogramming and epigenetics
Cellular Agriculture
Coordinator
Goals
The Stem Cell Engineering Research Group (SCERG)
SCERG’s vision is to be a leader in Regenerative Medicine, Cancer Therapies, Tissue Engineering and Cellular Agriculture, aiming at translating pioneering research and development in stem cell, biomaterials, tissue and organ engineering to improve human health. Its mission is to perform ground-breaking research translated into therapeutic and food applications.
SCERG’s goals are to develop innovative production systems for the generation and expansion of stem cells and their controlled differentiation into specific high quality cell types, as well as for the production of microtissues, organoids, and cell-derived biomolecules; and to design new platforms, including bioreactors, scaffolds, bioprinting and bioprocesses. Overall, with these goals, SCERG addresses the current challenges in Regenerative and Precision Medicine, Biopharmaceutical Industry and Cellular Agriculture to improve the quality of human wellbeing with high impact in the healthcare and food sectors.
SCERG relies on a cross-disciplinary strategy combining Bioprocess Engineering with Stem Cell Biology with activities focused on three Focus Areas: (A) Biology and Engineering of Pluripotent Stem Cells; (B) Development and Manufacturing of Cellular & Molecular Therapies; and (C) Devices and Materials Engineering for Health and Sustainability.
Focus Areas
Publications
Projects
People
The SCERG Team
For applications or further information, please get in touch!